Stem Cell–Derived Endothelial Cells
The inner blood-retina barrier (iBRB) plays a critical role in maintaining the selective environment of the retina by tightly regulating the movement of molecules between the bloodstream and neural tissue. Stem cell–derived endothelial cells offer a powerful tool to model and study this barrier in vitro.We generate endothelial cells from human stem cells to model the inner blood-retina barrier in vitro.
These stem cell–derived endothelial cells exhibit key features of retinal microvascular endothelium, such as tight junctions and selective permeability, making them ideal for studying retinal vascular health, disease mechanisms, and drug transport.
Stem Cell Technology and Retinal organoid
The aim of the project to generate functional retinal organoids. New developments in retinal organoid technology provide avenues for in vitro models of human retinal diseases, studies of pathological mechanisms, and development of therapies for retinal degeneration, including electronic retinal implants and gene therapy. Moreover, these innovations have played key roles in establishing models for large-scale drug screening, studying the stages of retinal development, and providing a human model for personalized therapeutic approaches, like cell transplants to replace degenerated retinal cells.
In order to understand the mechanisms underlying retinogenesisis and retinal organoid functionality, we culture mouse embryonic stem cells and differentiate these cells into 3D retinal organoid models based on a protocol that havedeveloped in our lab.
Disease Pathology
We are investigating the role of complement activation in retinal pigment epithelial (RPE) cells and choroidal endothelial cells (CEC) derived from iPSC lines generated from patients with age-related macular degeneration (AMD). The goal is to understand the cellular and molecular mechanisms contributing to AMD pathogenesis. In parallel, we are exploring the effects of specific genetic risk loci in RPE cells using a comprehensive multi-omics approach, including transcriptomics, proteomics, and epigenomics. Additionally, we are examining how choroidal cells respond to systemic and local aberrant complement activation, with a focus on identifying key pathways and molecular signatures that drive AMD progression. Together, these projects aim to elucidate the interplay between genetic predisposition and complement dysregulation, ultimately providing insights into novel therapeutic targets for AMD.


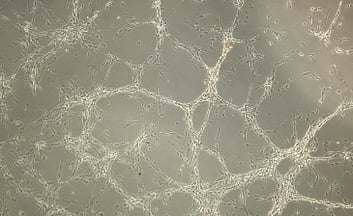

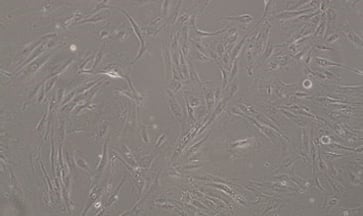
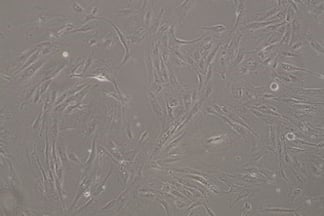
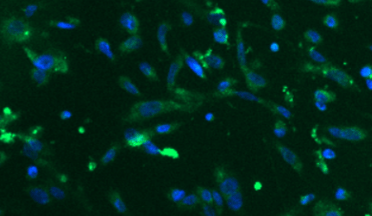
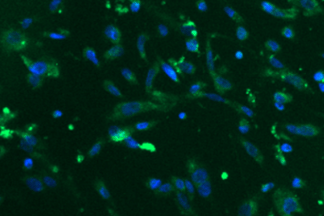
Stem Cell–Derived RPE for age related macular degeneration
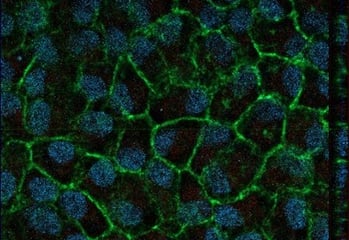
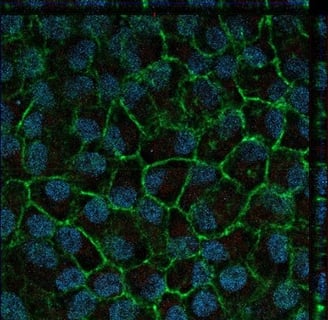
Contacts
zohreh.hosseinazdeh@radboudumc.nl
Socials
https://orcid.org/0000-0003-3407-7754
